Products intended for the treatment of acne and other skin conditions could break down into cancer-causing substances when improperly stored, according to a report in the Journal of Investigative Dermatology.
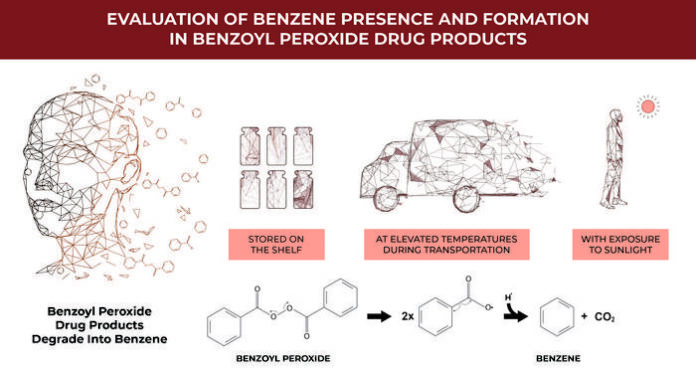
Topical treatments for both acne and rosacea that contain benzoyl peroxide (BPO) can degrade into the known carcinogen benzene if they are stored at room temperature or higher. Exposure to ultraviolet rays, including those found in sunlight, can also trigger the products’ transformation into a carcinogen, according to the study.
Degrading Acne Treatments May Increase Cancer Risks
People with acne or rosacea tend to use topical treatments daily for long periods of time, which increases their risk. Encapsulating the products does not appear to prevent them from breaking down into benzene. However, transporting and storing them at colder temperatures could prevent the chemical reaction.
“Our research demonstrates that BPO products can generate benzene at typical room and store shelf temperatures, while cold storage significantly reduces this formation,” said Christopher G. Bunick, a physician and researcher at Yale University School of Medicine, in a press release.
“These findings suggest a need to recommend refrigeration of BPO products throughout the supply chain — from manufacturing to patient use — to limit benzene exposure. Until formulations are developed to prevent benzene formation, refrigeration may serve as a practical solution to minimize unnecessary exposure. Additionally, dermatologists should continue to advise patients on the appropriate use of BPO, including potential risks associated with UV exposure.”
Researchers used mass spectrometry methods to detect benzene in 111 new, unopened products stored at room temperature on shelves of major U.S. retailers. They also examined the stability of the products with and without UV exposure.
Read More: The Causes of Adult Acne and How to Get Rid of It
A Public Health Concern
The products’ propensity to break down under relatively normal conditions represents a “potentially serious public health risk,” David Light, the study’s lead investigator and a pharmacy professor at Long Island University in New York, said in a press release.
The researchers recommend studies that look for actual cancer cases that could be connected to the use of these products. They add that topical acne treatments join a list of other consumer products that have raised safety concerns, including deodorants contaminated with benzene and shampoos containing phthalates.
These products receive less regulation from the US FDA than over-the-counter medications and far less than prescription drugs.
Read More: These 5 Supplements Can Keep Your Skin Healthy and Glowing
Article Sources
Our writers at Discovermagazine.com use peer-reviewed studies and high-quality sources for our articles, and our editors review for scientific accuracy and editorial standards. Review the sources used below for this article:
Before joining Discover Magazine, Paul spent over 20 years as a science journalist, specializing in U.S. life science policy and global scientific career issues. He began his career in newspapers, but switched to scientific magazines. His work has appeared in publications including Science News, Science, Nature, and Scientific American.
Source : Discovermagazine